News All News
February 23rd, 2022
Cantex licenses IP from Harvard to develop repurposed drug identified by Wyss Institute to treat inflammatory lung diseases including COVID-19
Organ Chip studies revealed azeliragon can reduce virus-associated inflammation in human lung tissue
By Lindsay Brownell, Wyss Institute
Boston/Cambridge, Mass., and Weston, Fla. - Cantex Pharmaceuticals, Inc., a clinical-stage pharmaceutical company based in Weston, Fla., and the Wyss Institute for Biologically Inspired Engineering at Harvard University announced today that Cantex has secured a global license from Harvard University’s Office of Technology Development (OTD) to develop azeliragon, a small-molecule drug in clinical development, into a treatment for inflammatory lung diseases including COVID-19.
Studies performed on the drug at the Wyss Institute in a Human Lung Alveolus Chip demonstrated that azeliragon significantly blocks the production of inflammation-causing cytokines including IL-6, IL-8, and IP-10 as well as RANTES, a key proinflammatory cytokine produced by virus-infected lung cells, following viral infection.
Based on these promising results in combination with extensive supporting literature from other studies, Cantex plans to conduct a Phase 2 clinical trial to test azeliragon in hospitalized patients with severe COVID-19, as well as Phase 2 trials in other pulmonary inflammatory diseases including chronic obstructive pulmonary disease (COPD) and steroid refractory asthma. The drug has previously been tested for Alzheimer’s disease and diabetic nephropathy, where it showed high levels of safety in Phase 3 clinical trials involving over 2,000 patients.
Cantex also plans to initiate Phase 2 clinical trials exploring the therapeutic effect of azeliragon in pancreatic and breast cancers, where RAGE has been implicated in disease progression as well as in complications of treatment.
“Monoclonal antibodies have made an important contribution to the treatment of COVID-19. However, their efficacy is limited, and they can be challenging to manufacture, store, distribute and administer. Thanks to the great work of the scientists at the Wyss Institute, we now have compelling evidence that azeliragon may have the potential to prevent severe COVID-19 illness in the form of a once-a-day pill. We’re excited to have the opportunity to conduct clinical trials of azeliragon for this disease, seeking to bring this groundbreaking therapy to patients to prevent the life-threatening inflammation that is the major cause of hospitalization and death from COVID-19 infection,” said Stephen Marcus, M.D., CEO of Cantex.
Rapid repurposing
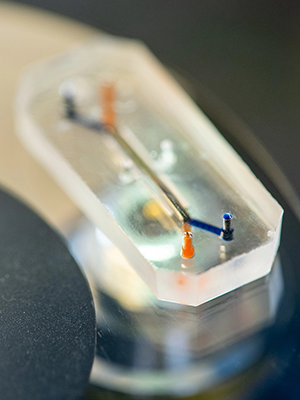
The Human Lung Alveolus Chip mimics the physiological conditions of the human lung, including the rhythmic motions of breathing, and replicates the lung's responses to drugs and disease more accurately than animal models. (Courtesy of the Wyss Institute.)
Azeliragon is a type of drug that binds to a protein on cells’ membranes called RAGE (receptor for advanced glycation endproducts) and inhibits its activity. When lung cells are damaged by viral or bacterial infection or airway irritants, they release molecules called “damage associated molecular patterns,” also known as DAMPs, that bind to and activate RAGE. RAGE is more highly expressed in the lung than in any other organ in the human body, and has been implicated as a major inflammatory mediator in several lung diseases, ranging from acute lung injury to COPD and asthma, all of which need better treatments. Azeliragon prevents RAGE from being activated by its normal binding partners, thus reducing inflammation. It is currently the only oral small molecule RAGE inhibitor in human clinical trials.
When SARS-CoV-2 appeared on the world stage in late 2019 and started wreaking havoc on patients’ lungs, researchers at the Wyss Institute in the lab of Founding Director Donald Ingber, M.D., Ph.D., immediately redirected their work to identifying existing drugs that could potentially be quickly repurposed to treat COVID-19.
As the Wyss team learned that the major cause of morbidity and mortality in patients with COVID-19 was related to the inflammatory “cytokine storm” that is triggered by SARS-CoV-2 infection, they focused their efforts on understanding how viral infection triggers rampant lung inflammation.
The Wyss team used its Human Lung Alveolus Chip, a microfluidic device containing human lung air sac and blood vessel cells that faithfully mimics human lung functions and disease states, to replicate viral infection in the lab. They applied cyclic stretching to the chip to mimic rhythmic breathing motions, a crucial element of human physiology that is absent from typical cells-in-a-dish experiments, and then introduced a virulent strain of influenza virus that is known to cause pneumonia and associated inflammation.
Infected cells in the Human Lung Alveolus Chips secreted high levels of inflammatory cytokines in response to the virus, much like the cytokine storm observed in COVID-19 patients. When the researchers introduced azeliragon into the chips, these cytokines were dramatically reduced. Harvard filed a U.S. provisional patent application for the use of azeliragon to treat pulmonary inflammatory diseases, and the business development teams at the Wyss and OTD started looking for a partner organization to help develop and commercialize it.
An easy pill to swallow
Although further studies would be needed to confirm the results, they were intriguing to Dr. Marcus at Cantex, as the company had previously acquired global rights to azeliragon and was pursuing its clinical development as a treatment for cancer and its associated complications. Just last month, independent research conducted by National Institute of Allergy and Infectious Disease (NIAID) scientists demonstrated that another small-molecule RAGE inhibitor called FPS-ZM1 can reduce inflammation and lung damage associated with COVID-19 in mice, although that compound has never been used in human clinical trials. With azeliragon's established track record of safety in human clinical trials and the Wyss Institute’s data showing that it could address virus-associated inflammation in human lung tissue, Cantex quickly moved to license the technology.
“When someone is hospitalized with respiratory symptoms due to COVID-19, the infection can, over several days, progress to the point where prolonged hospitalization or admission to an intensive care unit is required, or where uncontrolled lung inflammation causes death. In developing azeliragon, Cantex seeks to interrupt this process, accelerate recovery, and prevent life-threatening complications with a convenient, well-tolerated, once-daily oral anti-inflammatory medication. Moreover, since persistent symptoms of COVID-19 infection known as ‘long COVID’ are believed to be caused by persistent inflammation, it is possible that extending a patient’s course of the drug over several weeks may also prevent long COVID,” said Dr. Marcus.
Following FDA submissions that include data from the Wyss' Organ Chips, Cantex is planning to initiate a Phase 2 clinical trial in patients hospitalized with severe COVID later this year.
“I am very proud of the speed with which our team at the Wyss Institute moved to address the COVID-19 crisis, and how mechanistic studies of viral infection-associated inflammation enabled by the novel capabilities of human Organ Chips led to the repurposing of azeliragon for this new application. I am also especially grateful to our Business Development team for finding the perfect commercialization partner in Cantex,” said Ingber, who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences. “We are eager to see this potentially lifesaving therapy enter clinical trials at a similarly rapid pace, and eventually be placed in the hands of patients so that it can help stem the tide of the ongoing global pandemic, and address other inflammatory diseases as well.”
Press Contacts
Wyss Institute for Biologically Inspired Engineering at Harvard University
Lindsay Brownell, lindsay.brownell@wyss.harvard.edu, +1 617-432-8266
Cantex Pharmaceuticals
Stephen Marcus, smarcus@cantex.com, +1 954-315-3660
Harvard Office of Technology Development
Caroline Perry, caroline_perry@harvard.edu, +1 617-495-4157
Tags: licensing, drug development
Press Contact: Kirsten Mabry | (617) 495-4157
