News All News
March 21st, 2016
Blavatnik Biomedical Accelerator at Harvard University announces license and collaboration agreement
Research alliance will advance small-molecule therapy for most common form of acute leukemia, targeting proteins involved in transcriptional regulation
Cambridge, Mass.—March 21, 2016—Harvard University has entered into an exclusive license and research collaboration agreement with Merck, known as MSD outside the United States and Canada, to further the development of small-molecule therapeutics for leukemia and other cancers. The novel compounds, developed in the laboratory of Harvard scientist Matthew Shair, Ph.D., offer an innovative approach to cancer treatment, targeting enzymes that regulate transcription.
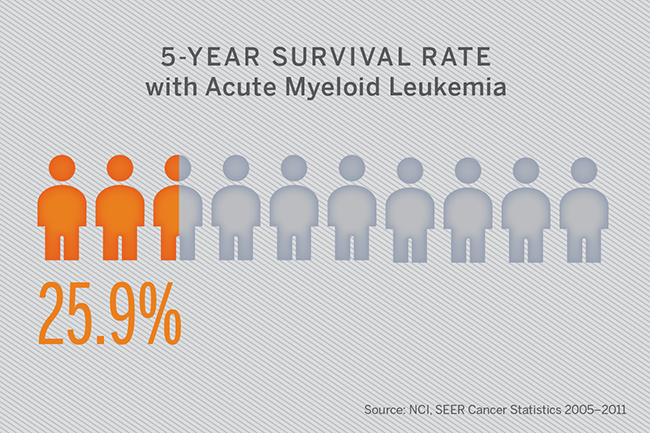
Acute myeloid leukemia (AML) is the most common form of acute leukemia, with an estimated 20,800 new cases in the United States in 2015. Moreover, AML accounts for the largest number of leukemia-related deaths, with a five-year overall survival rate of only approximately 26 percent.
Shair, a professor in Harvard’s Department of Chemistry and Chemical Biology, has discovered a novel therapeutic strategy for the treatment of AML—namely, to inhibit enzymes that regulate the transcription of key genetic programs that are altered in AML and other cancers. With support from Harvard’s Blavatnik Biomedical Accelerator, Shair’s laboratory has developed highly selective and potent small molecules, with favorable pharmaceutical properties, that are now poised for advancement toward clinical trials.
Merck will take over development of the candidate therapeutics and will engage in a research collaboration with the Shair laboratory to further investigate the biology of transcriptional regulator enzymes.
“Accelerator funding over the course of several years has enabled my laboratory to advance some of our experimental compounds to a relatively late stage of preclinical development,” said Shair. “It’s gratifying to have discovered a new biological target in the fight against AML, but even more fulfilling to have created a promising weapon against it.”

Matthew Shair, Professor of Chemistry and Chemical Biology, in his Harvard laboratory. (Photo by Stephanie Mitchell/Harvard Staff Photographer.)
Under the terms of the license agreement, Merck will pay to Harvard an upfront fee of $20 million and will be responsible for development, including clinical development, and for worldwide commercialization of products. The University is also eligible to receive development and commercialization milestone payments, as well as tiered royalties on any resulting products. The compensation, which acknowledges the value Harvard and the Blavatnik Biomedical Accelerator have created, will support future groundbreaking research.
“This recent agreement with the Shair laboratory is rooted in our belief that collaboration is the cornerstone for improving cancer care and driving innovation. It is the partnership among industry and academia that is truly critical to transforming cancer treatment and advancing the care for patients with difficult to treat blood cancers, such as acute myeloid leukemia,” said Dr. Eric Rubin, vice president and therapeutic area head, oncology early-stage development, Merck Research Laboratories.
The Blavatnik Biomedical Accelerator, administered by Harvard’s Office of Technology Development, supports the University’s innovative, early-stage life science research to promote the successful development of important biomedical advances that will improve health care and benefit society.
“University researchers bring a great degree of creativity and innovation to the toughest challenges in human medicine,” said Isaac T. Kohlberg, Harvard’s Senior Associate Provost and Chief Technology Development Officer. “Professor Shair’s inventive leukemia research, with funding from the Blavatnik Biomedical Accelerator, has reached a stage of development that is unusual in most universities but of great interest to the healthcare industry and ultimately to patients. His work could change the way clinicians treat a major disease.”
About Harvard University’s Office of Technology Development
Harvard’s Office of Technology Development (OTD) promotes the public good by fostering innovation and translating new inventions made at Harvard University into useful products that are available and beneficial to society. Our integrated approach to technology development comprises sponsored research and corporate alliances, intellectual property management, and technology commercialization through venture creation and licensing. Harvard OTD also manages the Blavatnik Biomedical Accelerator and the Physical Sciences & Engineering Accelerator. For more information, please visit http://otd.harvard.edu.
About the Blavatnik Biomedical Accelerator
The Blavatnik Biomedical Accelerator at Harvard University bridges the gap between the innovative life science research at Harvard and the successful development of high-impact biomedical products. The program provides essential project-based funding and expertise in technology development and business to help advance the University’s most promising biomedical technologies to more mature stages of development. In doing so, the Accelerator catalyzes the formation of new industry partnerships that will bring forth important biomedical discoveries, creating value and benefiting society.
Tags: Chemistry , licensing , sponsored research , therapeutics , Blavatnik Biomedical Accelerator
Press Contact: Kirsten Mabry | (617) 495-4157
