Novel adjuvants to enhance adaptive immune response of vaccines
Researchers in the lab of Ulrich von Andrian have discovered a novel adjuvanting behavior of a clinically approved and well-tolerated class of medicines known as bisphosphonates. The team is hoping to collaborate with manufacturers of COVID-19 vaccines to further demonstrate enhanced immune responses to infectious disease antigens.
Despite decades of adjuvant research, only four adjuvants have been approved in the seven major markets. Of these, only two (Alum and AS04) are licensed in the United States. Any additional adjuvants would need to meet stringent safety requirements imposed by the regulatory authorities. Although Alum is safe and efficacious, it has proven unsuccessful in several infectious disease applications. To address this unmet need, the lab of Dr. von Andrian began to explore alternatives to the traditional adjuvant modalities.
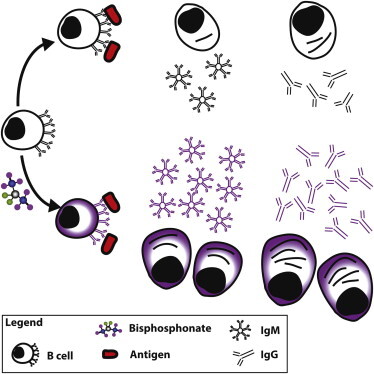
The discovery was made when the team noticed an increase in findings relating clodronate liposomes to antiviral immune responses. When they investigated deeper, the team discovered that bisphosphonates alone lead to enhanced and accelerated humoral immune responses to a wide variety of antigens including viruses, proteins, haptens, and commercial vaccine formulations. The team also found that bisphosphonates directly target B cells, leading to increased antibody production. Taken together, these findings represent a novel adjuvant class, distinct from traditional adjuvants that utilize pathways such as Toll-like receptor or inflammasome signaling or local macrophage depletion.
This work was published in Cell Reports.
Researchers in the lab of Ulrich von Andrian have discovered a novel adjuvanting behavior of a clinically approved and well-tolerated class of medicines known as bisphosphonates. The team is hoping to collaborate with manufacturers of COVID-19 vaccines to further demonstrate enhanced immune responses to infectious disease antigens.
Despite decades of adjuvant research, only four adjuvants have been approved in the seven major markets. Of these, only two (Alum and AS04) are licensed in the United States. Any additional adjuvants would need to meet stringent safety requirements imposed by the regulatory authorities. Although Alum is safe and efficacious, it has proven unsuccessful in several infectious disease applications. To address this unmet need, the lab of Dr. von Andrian began to explore alternatives to the traditional adjuvant modalities.
The discovery was made when the team noticed an increase in findings relating clodronate liposomes to antiviral immune responses. When they investigated deeper, the team discovered that bisphosphonates alone lead to enhanced and accelerated humoral immune responses to a wide variety of antigens including viruses, proteins, haptens, and commercial vaccine formulations. The team also found that bisphosphonates directly target B cells, leading to increased antibody production. Taken together, these findings represent a novel adjuvant class, distinct from traditional adjuvants that utilize pathways such as Toll-like receptor or inflammasome signaling or local macrophage depletion.
This work was published in Cell Reports.
Intellectual Property Status: Patent(s) Pending