News All News
March 15th, 2018
Startup promises minimally invasive heart repair
HoliStick Medical licenses surgical catheter technology from Harvard and collaborators for further development
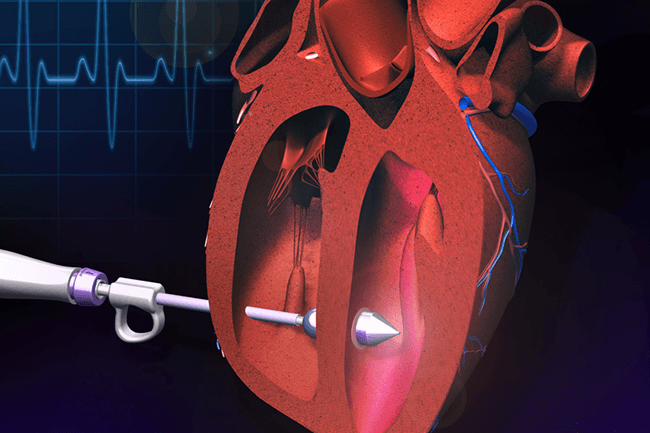
The technology is a catheter-like surgical device designed to repair holes in the heart, or tissue defects in other organs, using deployable soft structures. (Image courtesy of Ellen Roche.)
Cambridge, Mass. - March 15, 2018 - A minimally invasive surgical device to be commercialized by a newly launched startup could fundamentally transform the way doctors correct organ defects. For patients with certain conditions such as a hole in the heart, the device might one day provide a lasting repair without the complications and risks common to more invasive types of surgery.
Harvard University’s Office of Technology Development (OTD) has established a licensing agreement with HoliStick Medical, granting the Paris-based startup exclusive worldwide rights to commercially develop this medical technology co-owned by Harvard, Brigham & Women’s Hospital, Boston Children’s Hospital, and MIT.
The enabling technology is a specialized catheter device that can repair holes in the heart, or tissue defects in other organs, using deployable soft structures. Designed to work with sticky and flexible materials such as patches, the device incorporates mechanisms to delicately close a hole without requiring the use of sutures or rigid devices. Critically, the device offers a non-invasive approach to tissue repair, facilitating clinicians’ access to difficult-to-reach organs, as demonstrated in successful animal studies and published in Science Translational Medicine (Roche et al., 2015).
The technology was created through a collaboration between bioengineers and clinicians working with the Harvard Biodesign Lab, led by Conor Walsh, PhD, who is the John L. Loeb Associate Professor of Engineering and Applied Sciences at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) and a Core Faculty Member at Harvard’s Wyss Institute for Biologically Inspired Engineering.
The lead scientist on the project was Ellen Roche, PhD, a doctoral student at SEAS, co-advised by Walsh and Prof. David Mooney, PhD. Roche has since joined the MIT faculty as an Assistant Professor in the Department of Mechanical Engineering and the Institute for Medical Engineering and Science.
Walsh and Roche were joined on the project by co-PIs Pedro del Nido, MD, Chief of Cardiac Surgery at Boston Children’s Hospital and Professor of Surgery at Harvard Medical School (HMS), and Jeffrey M. Karp, PhD, a principal investigator at Brigham & Women’s Hospital and Professor of Medicine at HMS, along with members of their research groups and other collaborators. Del Nido is also principal investigator of the Boston Pediatric Device Consortium, an FDA-funded resource for inventors developing devices designed for children.
Walsh’s lab at SEAS and the Wyss Institute is known for combining elements of engineering, industrial design, medical practice, and business savvy to design novel robotic systems and smart medical devices that address human needs.
“Part of our lab’s goal is to develop disruptive technologies that augment or restore human performance, and in this case we’ve developed a technology that augments a doctor’s ability to perform a medical procedure,” said Walsh. “Ellen’s brilliant leadership of this project and the essential input of our engineering and clinical colleagues have resulted in the creation of a less invasive, less traumatic device that could really improve the way difficult tissue repairs are performed and, hopefully, reduce the need for procedures like open-heart surgery.”
HoliStick is backed by Truffle Capital, a French venture capital firm that focuses on disruptive startups in life sciences and information technology, with $1.2 billion in assets under management.
“To carry an early-stage medical technology into clinical application requires both commitment and strategic sophistication,” said Sam Liss, Executive Director for Strategic Partnerships in Harvard OTD. “We’re thrilled to see this technology enter commercial development in the hands of a well-resourced startup that shares our vision of advancing innovation to transform patient care worldwide.”
Research to develop and evaluate the catheter device at Harvard and collaborating institutions was supported in part by the National Institutes of Health and the National Science Foundation.
Tags: medical devices, Harvard startups
Press Contact: Kirsten Mabry | (617) 495-4157
